Types of Membrane Filtration Based on Pore Size
The wide spectrum of filtration challenges, from removing large suspended solids to separating individual ions, necessitates a range of membrane technologies. These technologies are primarily distinguished by their characteristic pore sizes, leading to a classification into four main types of membrane filtration: Microfiltration, Ultrafiltration, Nanofiltration, and Reverse Osmosis. Each type offers a specific level of separation and is suited for distinct applications.
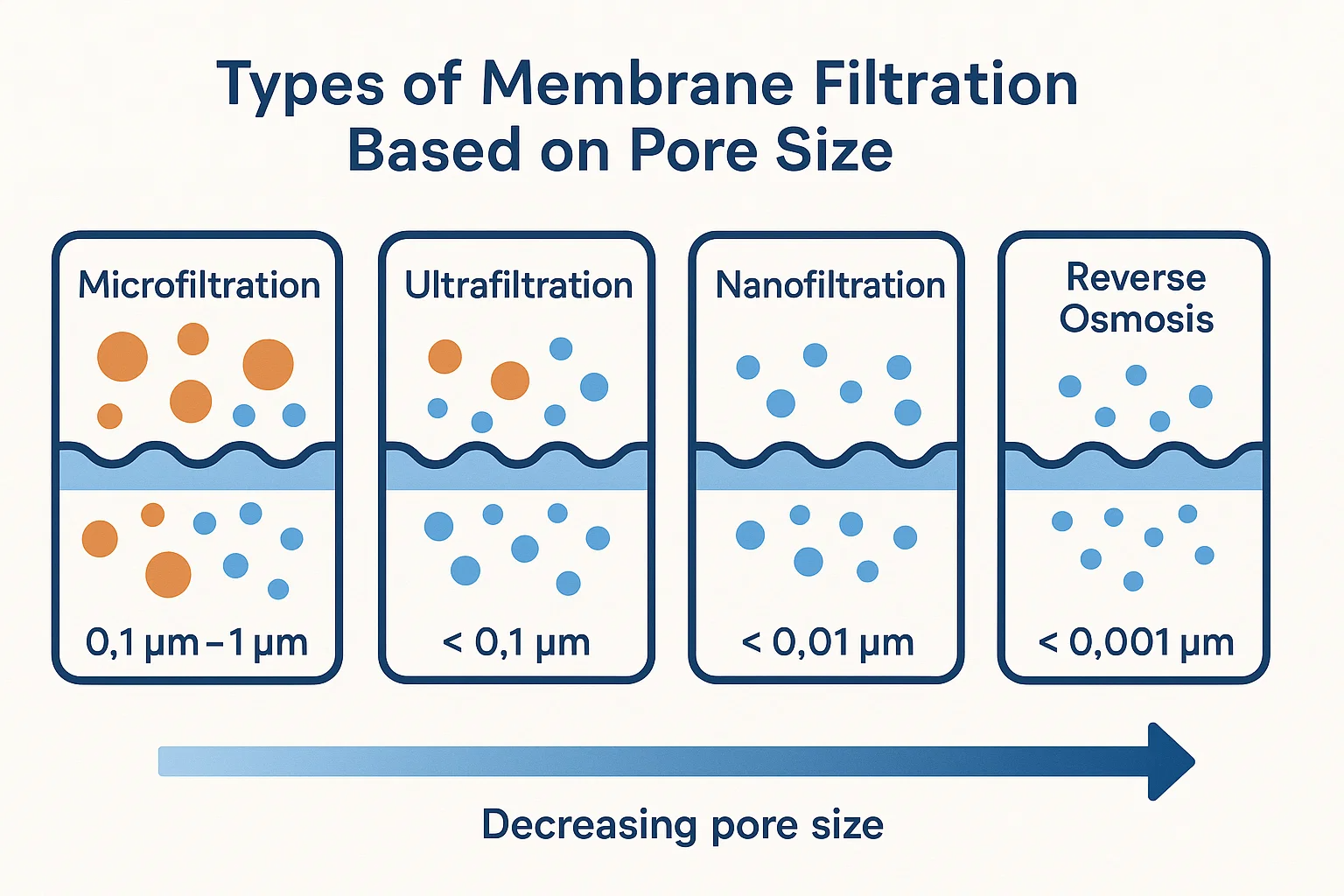
Microfiltration (MF)
Microfiltration (MF) represents the coarsest end of membrane filtration. MF membranes are designed to remove suspended solids, bacteria, and large colloids from liquids or gases.
- Pore Sizes: Typically range from 0.1 to 10 microns (µm) . common and widely used pore sizes: 0.22 µm, 0.45 µm, 0.8 µm, and 1.0 µm
Standardization: Many regulatory guidelines and industry standards (e.g., for water quality testing, pharmaceutical manufacturing) specify the use of certain pore sizes, particularly 0.22 µm and 0.45 µm.
- Typical Applications:
- Water Treatment: Removal of suspended solids, turbidity, and protozoa (like Giardia and Cryptosporidium ) from drinking water. Used as a pre-treatment for other membrane processes (UF, NF, RO).
- Food and Beverage: Clarification of fruit juices, wine, and beer; removal of yeast and bacteria in dairy processing.
- Pharmaceuticals: Sterilization of cold liquids, clarification of biological solutions.
- Biotechnology: Cell harvesting, separation of biomass.
-
0.22 µm:
- "Sterilizing Grade": This is the gold standard for sterile filtration . Most bacteria are larger than 0.22 µm, so a filter with this pore size is generally considered effective for removing bacteria and ensuring sterility in liquids. This is crucial in pharmaceuticals, biotechnology (e.g., cell culture media preparation), and for producing sterile water.
- It's important to note that while it removes most bacteria, some very small bacteria (like Mycoplasma ) and viruses can pass through.
-
0.45 µm:
- General Microbiological Filtration: This pore size is widely adopted for microbiological analysis , including water testing and food/beverage quality control. It's excellent for capturing most common bacteria for enumeration (counting colonies) because it allows for good nutrient diffusion through the pores, supporting robust bacterial growth on the filter surface after filtration.
- Clarification: It's also frequently used for general clarification of solutions to remove particulates, larger microorganisms, and turbidity, without necessarily achieving full sterility.
-
0.8 µm:
- Particle Removal and Pre-filtration: Often used for coarser particle removal and as a pre-filter to protect finer membranes (like 0.45 µm or 0.22 µm filters) from premature clogging by larger debris.
- Specific Microbiological Applications: Sometimes used for specific microbiological assays or particle monitoring where larger particles or specific types of cells need to be retained, while allowing smaller components to pass. Common in air monitoring (e.g., asbestos analysis) and some fluid analyses.
-
1.0 µm:
- Coarse Filtration/Pre-filtration: Generally used for coarse filtration to remove larger suspended solids, sediment, and gross particulates from liquids. This is a common pre-filtration step in many industrial and laboratory processes to extend the life of subsequent finer filters.
- Cell Harvesting/Clarification: Can be used in some biological applications for harvesting larger cells or clarifying highly turbid solutions.
Ultrafiltration (UF)
Ultrafiltration (UF) operates at a finer scale than microfiltration, capable of removing smaller particles and macromolecules. UF membranes typically retain viruses, proteins, and larger organic molecules, while allowing water and smaller dissolved salts to pass through.
- Pore Sizes: Range from 0.01 to 0.1 microns (µm) , or often expressed as Molecular Weight Cut-Off (MWCO) from 1,000 to 500,000 Daltons. MWCO refers to the approximate molecular weight of the smallest globular protein that is 90% retained by the membrane.
- Typical Applications:
- Water Treatment: Removal of viruses, endotoxins, colloids, and macromolecules for drinking water purification; wastewater treatment for reuse.
- Food and Beverage: Concentration of milk proteins, clarification of juices, recovery of enzymes.
- Pharmaceuticals & Biotechnology: Concentration and purification of proteins, enzymes, and vaccines; removal of pyrogens.
- Industrial: Oil/water emulsion separation, paint recovery in electrocoat processes.
Nanofiltration (NF)
Nanofiltration (NF) membranes are often referred to as "loosely rejecting RO membranes" because they fall between UF and RO in terms of separation capabilities. NF membranes are effective at removing multivalent ions (like hardness ions), some smaller organic molecules, and most viruses, while allowing monovalent ions (like sodium chloride) and water to pass more freely than RO membranes.
- Pore Sizes: Range from 0.001 to 0.01 microns (µm) , or MWCO typically from 150 to 1,000 Daltons.
- Typical Applications:
- Water Softening: Removal of hardness (calcium, magnesium) from water without requiring chemical regeneration.
- Drinking Water: Removal of color, pesticides, and dissolved organic carbon (DOC).
- Food and Beverage: Demineralization of whey, sugar refining, product concentration.
- Pharmaceuticals: Antibiotic concentration, desalting.
- Industrial: Dye removal from wastewater, separation of specific components in chemical processes.
Reverse Osmosis (RO)
Reverse Osmosis (RO) represents the finest level of membrane separation, capable of rejecting virtually all dissolved salts, inorganic molecules, and larger organic molecules. It works by applying pressure greater than the osmotic pressure, forcing water through an extremely dense membrane while leaving dissolved impurities behind.
- Pore Sizes: Effectively < 0.001 microns (µm) , or non-porous in the traditional sense, operating more on a solution-diffusion mechanism. They primarily reject based on charge and size, effectively removing ions.
- Typical Applications:
- Desalination: Conversion of seawater or brackish water into potable water.
- Ultrapure Water Production: Manufacturing of high-purity water for electronics, pharmaceuticals, and power generation.
- Wastewater Treatment: High-level purification for water reuse and discharge.
- Food and Beverage: Concentration of fruit juices, production of deionized water.
- Industrial: Process water purification, product recovery.
| Filtration Type | Typical Pore Size Range | Key Separations | Typical Operating Pressure (bar/psi) | Common Applications |
| Microfiltration (MF) | 0.1 to 10 µm | Suspended solids, bacteria, large colloids, algae | 0.1 - 2 bar (1.5 - 30 psi) | Water purification (pre-treatment), food/beverage clarification, pharmaceutical cold sterilization, bioreactor filtration |
| Ultrafiltration (UF) | 0.01 to 0.1 µm (or 1,000 to 500,000 MWCO) | Viruses, proteins, macromolecules, endotoxins, colloids | 0.5 - 7 bar (7 - 100 psi) | Drinking water treatment, protein concentration, wastewater reuse, enzyme purification, paint recovery |
| Nanofiltration (NF) | 0.001 to 0.01 µm (or 150 to 1,000 MWCO) | Divalent and multivalent ions (e.g., calcium, magnesium), some organic molecules, pesticides, viruses | 5 - 30 bar (70 - 450 psi) | Water softening, removal of color and organics, demineralization of food products, wastewater treatment |
| Reverse Osmosis (RO) | < 0.001 µm (or non-porous; ion rejection) | Virtually all dissolved salts (ions), small inorganic molecules, organic molecules, bacteria, viruses | 10 - 70 bar (150 - 1000 psi) | Desalination of seawater/brackish water, ultrapure water production, high-level wastewater purification, pharmaceutical ingredient concentration |
More Related:
Introduction to Membrane Filters and Pore Size
Membrane filters are sophisticated separation tools that have revolutionized various industries, from water purification to pharmaceuticals. At their core, these filters function by acting as selective barriers, allowing certain substances to pass through while retaining others. The effectiveness of a membrane filter in performing this critical task hinges almost entirely on one crucial characteristic: its pore size .
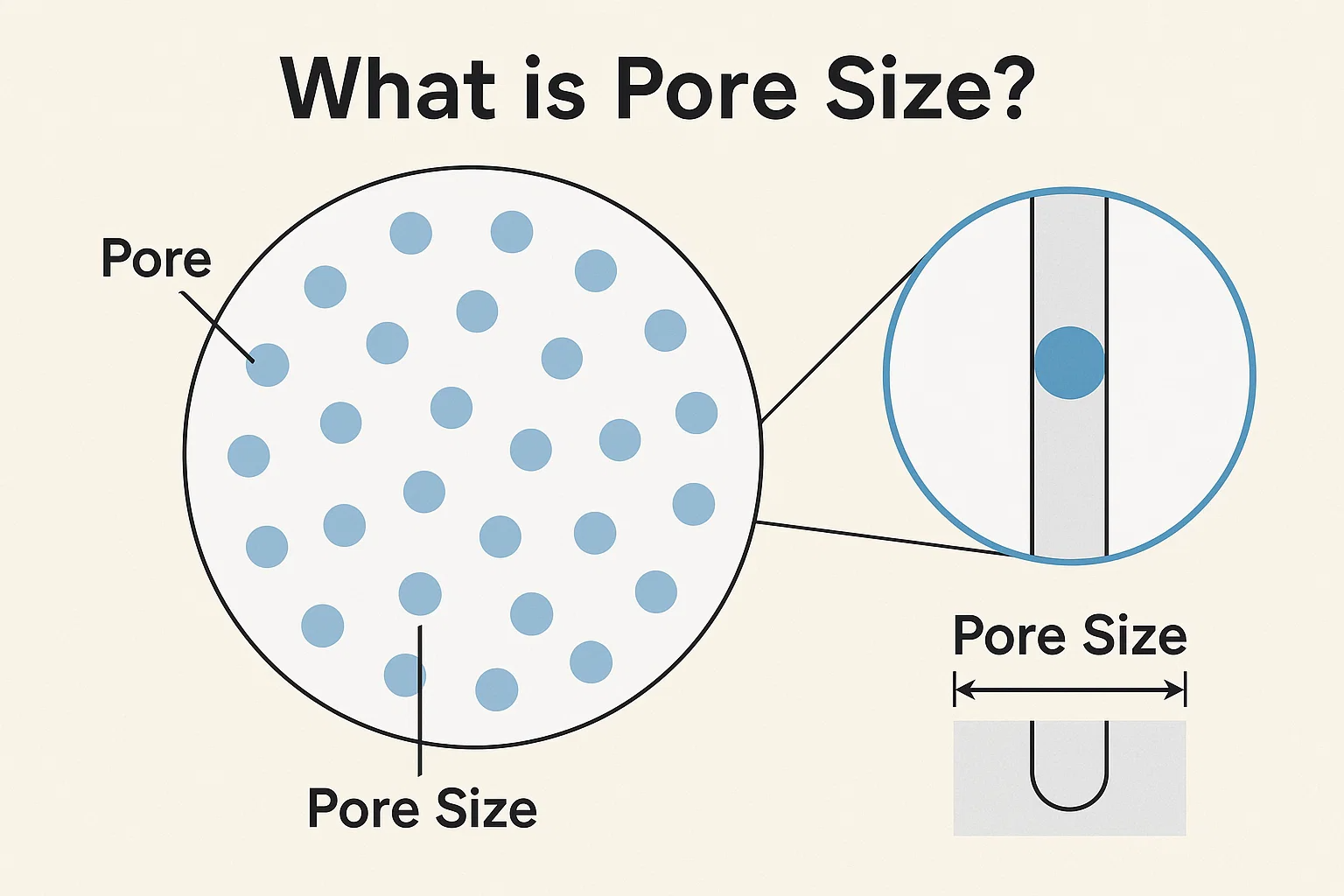
The pore size of a membrane filter dictates which particles, molecules, or even ions can be separated from a fluid stream. Imagine a microscopic sieve; the size of the holes in that sieve determines what passes through and what gets caught. Similarly, the miniscule pores within a membrane filter are engineered to specific dimensions to achieve desired separation outcomes.
Understanding membrane pore size is paramount in filtration processes. An incorrectly chosen pore size can lead to inefficient filtration, premature membrane fouling, or even damage to the membrane itself. Conversely, selecting the optimal pore size ensures efficient separation, extends membrane lifespan, and ultimately leads to more effective and economical processes.
Now let's delve into the intricate world of membrane filter pore size. We will define:
* what pore size truly means
* explore the different categories of membrane filtration based on pore size
* discuss the factors influencing pore size selection
* highlight the diverse applications where these filters are indispensable.
* Furthermore, we will examine methods for determining pore size, address common challenges, and look at the exciting trends shaping the future of membrane technology.
What is Pore Size?
At the heart of every membrane filtration process lies the concept of pore size . In the context of membrane filters, pore size refers to the average diameter of the microscopic openings or channels that permeate the membrane material . These pores are not simply holes, but rather intricate pathways designed to allow the passage of fluids while physically blocking particles larger than their defined dimensions.
The units of measurement for pore size are typically expressed in either microns (µm) or nanometers (nm) . To put these units into perspective:
- 1 micron (µm) is one-millionth of a meter ( 1 0 − 6 meters). For comparison, a human hair is roughly 50-100 µm in diameter.
- 1 nanometer (nm) is one-billionth of a meter ( 1 0 − 9 meters). A single water molecule is approximately 0.27 nm in diameter.
The choice of unit often depends on the scale of filtration. Microns are commonly used for larger pore sizes found in microfiltration, while nanometers are more prevalent when discussing the extremely fine pores of ultrafiltration, nanofiltration, and reverse osmosis membranes.
The profound impact of pore size on filtration efficiency cannot be overstated. It directly dictates the cut-off point for separation. Imagine a membrane with a pore size of 0.2 µm. This membrane is designed to retain any particle or microorganism larger than 0.2 µm, while allowing smaller molecules and water to pass through.
- Smaller pore sizes generally lead to higher filtration efficiency, as they can remove finer particles, dissolved solids, and even some viruses. However, this often comes at the cost of reduced flux (flow rate) and increased pressure drop across the membrane, as the resistance to flow is higher.
- Larger pore sizes allow for higher flux and lower pressure requirements, making them suitable for removing coarser particles or for pre-filtration steps. The trade-off, however, is a lower degree of separation and the inability to remove very fine contaminants.
Therefore, the careful selection of a membrane's pore size is a critical design parameter, directly correlating to the desired level of purity and the operational efficiency of the filtration system. It's a delicate balance between achieving the necessary separation and maintaining a practical flow rate for the given application.
Factors Affecting Pore Size Selection
Choosing the correct membrane filter pore size is a critical decision that directly impacts the success, efficiency, and cost-effectiveness of any filtration process. This selection is not arbitrary; it's a careful balancing act influenced by several key factors that dictate the required separation, membrane compatibility, and operational feasibility.
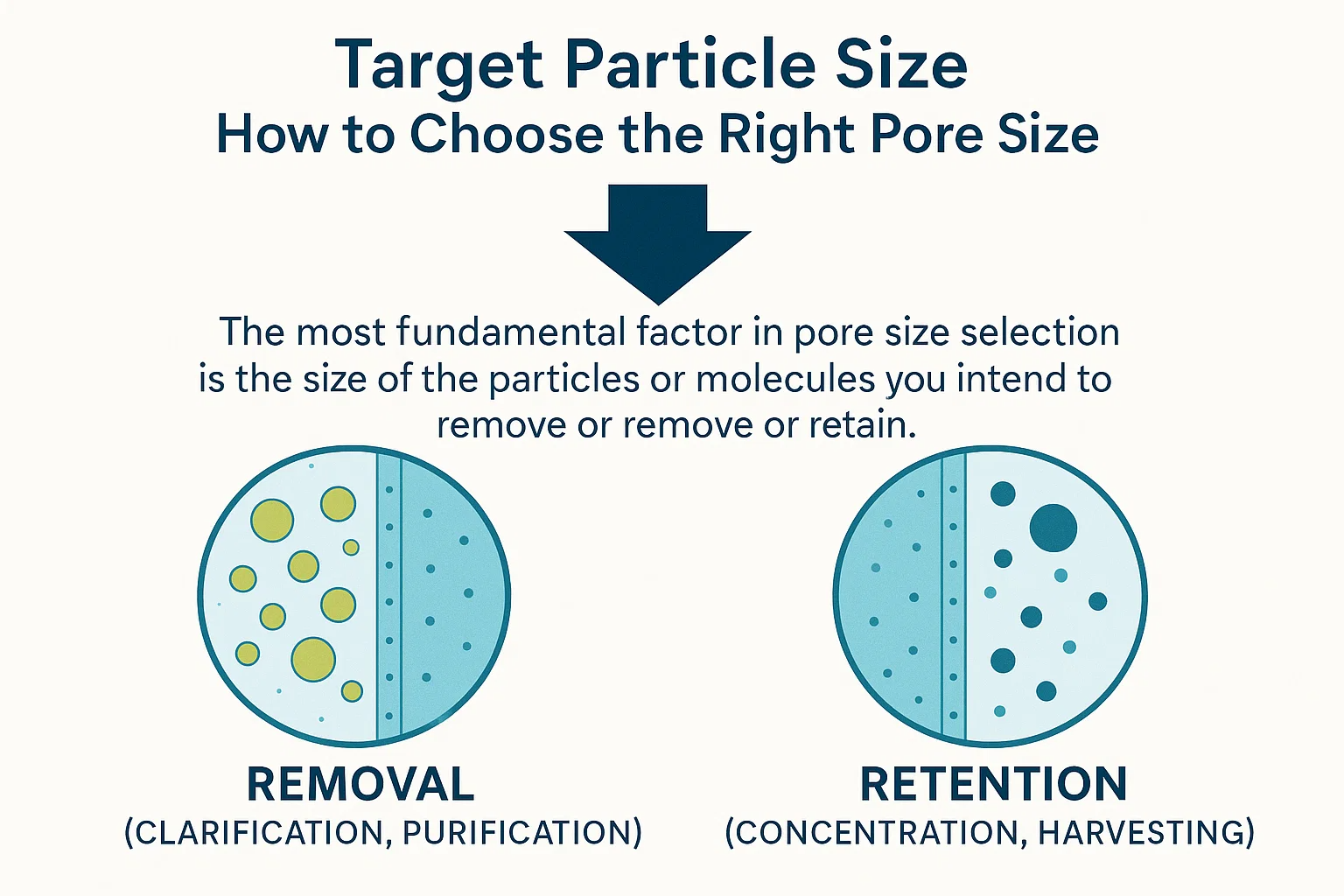
Target Particle Size: How to Choose the Right Pore Size
The most fundamental factor in pore size selection is the size of the particles or molecules you intend to remove or retain .
- For removal (clarification, purification): The membrane pore size must be significantly smaller than the target contaminant. For example, if you need to remove bacteria with an average size of 0.5 µm, you would likely select a microfiltration membrane with a pore size of 0.2 µm or smaller to ensure effective retention. A common rule of thumb is to choose a pore size 1/3 to 1/10 the size of the smallest particle you wish to remove, accounting for particle shape and potential membrane fouling.
- For retention (concentration, harvesting): Conversely, if your goal is to concentrate a desired substance (e.g., proteins or cells), the membrane pore size should be small enough to retain the target substance while allowing the solvent and smaller impurities to pass through. This is where the concept of Molecular Weight Cut-Off (MWCO) becomes particularly relevant for UF and NF membranes.
Understanding the size distribution of the components in your fluid stream is paramount. This often requires prior analysis of the feed stream using techniques like dynamic light scattering or microscopy.
Membrane Material: Influence on Pore Size and Compatibility
The material from which a membrane is constructed plays a significant role in its inherent pore structure, chemical resistance, and overall performance. Different materials lend themselves to different pore size ranges and applications:
-
Polymeric Membranes: These are the most common type and include materials like polysulfone (PS), polyethersulfone (PES), polyvinylidene fluoride (PVDF), cellulose acetate (CA), polyamide (PA), and polypropylene (PP).
- Influence on Pore Size: The manufacturing process (e.g., phase inversion, stretching) and the polymer itself dictate the achievable pore size range and distribution. For instance, cellulosic membranes are often used for general filtration where hydrophilic properties are desired, while PVDF is known for its chemical resistance and broad pore size availability. Polyamide is the dominant material for RO and NF membranes due to its excellent salt rejection properties.
- Compatibility: The chemical compatibility of the membrane material with the feed fluid (pH, solvents, oxidizers) and cleaning chemicals is crucial. Using an incompatible material can lead to membrane degradation, changes in pore size, and system failure. Temperature limitations of the material also influence suitability.
-
Ceramic Membranes: Made from materials like alumina, zirconia, or titania, these membranes are typically more robust.
- Influence on Pore Size: Ceramic membranes generally offer very uniform pore sizes, making them suitable for precise separations. They are commonly found in MF and UF applications.
- Compatibility: They exhibit exceptional chemical and thermal stability, allowing them to withstand harsh chemical environments, high temperatures, and aggressive cleaning regimes that polymeric membranes cannot.
Operating Conditions: Pressure, Temperature, and Flow Rate
The conditions under which the filtration process operates also heavily influence pore size selection and membrane performance.
- Pressure: As discussed, a higher driving pressure is required to overcome the increased hydraulic resistance of smaller pores. The membrane chosen must be able to withstand the necessary operating pressure without compacting or sustaining damage. Insufficient pressure will lead to low flux, while excessive pressure can damage the membrane structure.
- Temperature: Temperature affects the viscosity of the fluid and, consequently, the flux through the membrane. Higher temperatures generally lead to lower fluid viscosity and thus higher flux. However, membrane materials have temperature limits, beyond which their structural integrity or pore size stability may be compromised.
- Flow Rate (Flux): The desired permeate flow rate (flux) is a critical design parameter. While smaller pores offer better separation, they inherently provide lower flux at a given pressure. System design must balance the need for separation with the required throughput. Higher flow rates might necessitate larger membrane surface areas or higher operating pressures, impacting capital and operating costs.
In summary, selecting the right membrane filter pore size is a multi-faceted decision that requires a thorough understanding of the feed characteristics, the desired separation outcome, the properties of available membrane materials, and the practical constraints of the operating environment. A misstep in this selection can lead to costly inefficiencies or even process failure.
Applications of Membrane Filters by Pore Size
The ability of membrane filters to precisely control what passes through and what is retained, largely due to their engineered pore sizes, makes them indispensable across a vast array of industries. From ensuring safe drinking water to manufacturing life-saving drugs, these filters are central to purification, separation, and concentration processes.
Water Filtration: Drinking Water, Wastewater Treatment
Membrane filters are cornerstones of modern water treatment, addressing purity challenges ranging from macroscopic contaminants to microscopic pathogens and dissolved salts.
- Microfiltration (MF) and Ultrafiltration (UF): These membranes, with pore sizes in the 0.1 to 10 µm (MF) and 0.01 to 0.1 µm (UF) range, are widely used for the removal of suspended solids, turbidity, bacteria, protozoa (like Cryptosporidium and Giardia ), and viruses from drinking water sources. They are excellent pre-treatment steps for more advanced membrane systems, protecting finer membranes from fouling. In wastewater treatment, MF/UF can produce high-quality effluent suitable for discharge or even reuse, by effectively removing suspended solids, bacteria, and some organic matter.
- Nanofiltration (NF): With pore sizes typically 0.001 to 0.01 µm , NF membranes are employed for water softening by removing multivalent hardness ions (calcium, magnesium) and for reducing levels of dissolved organic carbon (DOC), color, and synthetic organic compounds (e.g., pesticides) from drinking water. This provides a higher quality permeate than UF.
- Reverse Osmosis (RO): Having effectively < 0.001 µm 'pore' sizes (operating via solution-diffusion), RO membranes are the ultimate barrier for water purification. They are critical for desalination of seawater and brackish water, producing potable water. RO is also essential for manufacturing ultrapure water required in industries like electronics, pharmaceuticals, and power generation, by removing nearly all dissolved salts and impurities.
Air Filtration: HVAC Systems, Cleanrooms
While the term "pore size" is usually associated with liquid filtration, the principle applies equally to air (gas) filtration, where membranes filter out airborne particulates.
- Microfiltration (MF) (and HEPA/ULPA media): Specialized membrane-like media, often classified by particle removal efficiency rather than discrete pore size, are used. For example, HEPA (High-Efficiency Particulate Air) filters typically capture 99.97% of particles 0.3 μ m in size, and ULPA (Ultra-Low Particulate Air) filters are even finer. These are crucial for:
- HVAC Systems: Improving indoor air quality by removing dust, pollen, mold spores, and some allergens.
- Cleanrooms: Creating and maintaining highly controlled environments (e.g., ISO Class 1 to 9) essential for semiconductor manufacturing, pharmaceutical production, and delicate research, where even sub-micron particles can cause contamination or defects.
Pharmaceuticals: Sterilization, Drug Development
The stringent purity requirements of the pharmaceutical industry make membrane filters indispensable.
- Microfiltration (MF): Sterile filtration of liquids (e.g., culture media, buffers, ophthalmic solutions) before packaging is a common application for 0.1 or 0.2 µm MF membranes, ensuring the removal of bacteria and fungi while avoiding heat-sensitive active ingredients.
- Ultrafiltration (UF): UF membranes (typically 0.01 to 0.1 µm or specific MWCOs) are vital for:
- Protein Concentration and Purification: Concentrating therapeutic proteins, enzymes, and vaccines.
- Diafiltration: Removing salts or exchanging buffers during protein purification.
- Pyrogen Removal: Eliminating endotoxins (pyrogens) from water for injection (WFI).
- Nanofiltration (NF) and Reverse Osmosis (RO): Used for pre-treatment of feed water for UF/RO systems, and for generating pharmaceutical-grade water (e.g., Purified Water, Water for Injection) which requires extremely low levels of impurities, including dissolved salts and organic compounds.
Food and Beverage: Clarification, Sterilization
Membrane filters enhance the quality, shelf-life, and safety of a wide range of food and beverage products.
- Microfiltration (MF):
- Beverage Clarification: Clarification of wine, beer (removing yeast, bacteria, and haze particles), and fruit juices.
- Dairy Processing: Cold pasteurization of milk (reducing bacterial load without heat), fractionation of milk components.
- Ultrafiltration (UF):
- Protein Concentration: Concentrating milk proteins (e.g., for cheese production), whey protein concentration.
- Juice Clarification: Removing suspended solids and macromolecules from juices while preserving flavor.
- Nanofiltration (NF):
- Sugar Refining: Desalting and purification of sugar solutions.
- Juice Concentration: Partial concentration of juices with simultaneous demineralization.
- Reverse Osmosis (RO):
- Concentration: Concentration of heat-sensitive liquids like coffee, fruit juices, or dairy products, offering energy savings compared to evaporation.
- Water for Processing: Providing high-purity water for product formulation and cleaning.
Industrial Applications: Chemical Processing, Oil and Gas
Beyond consumables, membrane filters address critical separation and purification needs in heavy industry.
- Microfiltration (MF) and Ultrafiltration (UF):
- Wastewater Treatment: General clarification and removal of suspended solids from industrial effluents.
- Emulsion Breaking: Separating oil from water in metalworking fluids or produced water in the oil and gas industry.
- Catalyst Recovery: Retaining valuable catalysts from reaction mixtures.
- Pre-treatment: Protecting other downstream equipment and finer membranes.
- Nanofiltration (NF) and Reverse Osmosis (RO):
- Process Water Purification: Providing high-purity water for boilers, cooling towers, and manufacturing processes.
- Product Recovery: Recovering valuable chemicals from waste streams.
- Brine Concentration: Concentrating salt solutions in various chemical processes.
- Chemical Separation: Separating specific components in chemical synthesis or purification steps.
How to Determine the Pore Size of a Membrane Filter
While the pore size is a fundamental characteristic of a membrane filter, it's not always a simple, direct measurement. Instead, it's often inferred through standardized testing or provided by manufacturers based on their quality control processes. Accurate pore size determination is crucial for ensuring the membrane performs as expected for its intended application.
Specifications Provided by Manufacturers
The most common way to know a membrane filter's pore size is by reviewing the technical specifications and data sheets provided by the manufacturer . Reputable manufacturers invest heavily in quality control and characterization of their products. These specifications will typically list:
- Nominal Pore Size: This is a general classification, indicating the average pore size. It means the membrane is designed to retain a certain percentage of particles at or above the stated size. For example, a 0.2 µm nominal filter might retain 99.9% of particles at that size. It's an average and doesn't imply every pore is exactly that size.
- Absolute Pore Size: This is a more precise specification, indicating that all particles larger than the stated size are retained (often 100% retention under specific test conditions). This is critical for applications like sterile filtration where complete removal of microorganisms is required.
- Molecular Weight Cut-Off (MWCO): For ultrafiltration and nanofiltration membranes, manufacturers often specify MWCO in Daltons, which describes the molecular weight at which 90% of a specific globular protein (or dextran) is retained by the membrane. This is a functional measure of pore size for molecular separations.
- Retention Ratings for Specific Organisms: Especially for pharmaceutical or water treatment applications, manufacturers might specify the membrane's ability to retain specific bacteria (e.g., Brevundimonas diminuta for 0.22 µm sterile filters) or viruses. This offers a practical, application-oriented measure of performance.
It's important to note that different manufacturers might use slightly different testing methodologies or definitions for "nominal" vs. "absolute," so comparing specifications across brands requires careful consideration.
Testing Methods: Bubble Point Test, Microscopic Analysis
Beyond manufacturer claims, there are established methods to characterize or verify the effective pore size and integrity of a membrane filter.
1. Bubble Point Test
The bubble point test is a widely used, non-destructive method for determining the largest pore size in a membrane filter, and for verifying membrane integrity. It's based on the principle that liquid held in a pore by surface tension can be forced out by gas pressure.
- Principle: The membrane is first wetted with a liquid (e.g., water or alcohol), filling all the pores. Gas pressure (usually air or nitrogen) is then applied to one side of the wetted membrane, while the other side is open to the atmosphere (or submerged in liquid). As the gas pressure gradually increases, it will eventually overcome the surface tension holding the liquid in the largest pore. At this "bubble point," a continuous stream of bubbles will be observed emerging from the wet side of the membrane.
- Calculation: The pressure at which this occurs is directly related to the largest pore size by the Young-Laplace equation:
-
- P=( 4γcosθ )/D:
- P is the bubble point pressure
- γ is the surface tension of the wetting liquid
- θ is the contact angle of the liquid with the pore wall (often assumed to be 0 ∘ for complete wetting, so cos θ = 1 )
- D is the diameter of the largest pore.
The bubble point test is excellent for quality control, detecting manufacturing defects, or verifying if a membrane has been damaged or compromised (e.g., by chemical attack or excessive pressure) in use. A lower-than-expected bubble point indicates larger pores are present, implying a loss of integrity.
2. Microscopic Analysis (e.g., Electron Microscopy)
For a more direct visual assessment of pore structure, advanced microscopic techniques can be employed, particularly:
- Scanning Electron Microscopy (SEM): SEM provides high-resolution images of the membrane surface and cross-section, allowing direct visualization of the pores. While it doesn't give a functional pore size like the bubble point test, it can reveal pore morphology, distribution, and overall membrane structure. Modern image analysis software can then be used to measure the size of visible pores and generate a pore size distribution.
- Transmission Electron Microscopy (TEM): TEM offers even higher magnification and resolution, useful for characterizing the very fine pores of UF, NF, and RO membranes, especially their internal structure.
While invaluable for research and development, microscopic analysis is typically a laboratory method and not a routine in-process or field test for pore size verification due to its complexity and cost.
Importance of Accurate Pore Size Determination
The precise determination of pore size is paramount for several reasons:
- Performance Assurance: Ensures the membrane will achieve the desired separation efficiency (e.g., sterility, clarity, solute rejection).
- Process Optimization: Helps in selecting the right membrane for a specific application, preventing over-filtration (too small pores, high cost, low flux) or under-filtration (too large pores, insufficient purity).
- Quality Control: Serves as a vital quality control measure for manufacturers and end-users, confirming batch consistency and product integrity.
- Troubleshooting: Aids in diagnosing issues like fouling, damage, or manufacturing defects that might alter the effective pore size.
In essence, understanding and verifying the pore size of a membrane filter is not just an academic exercise; it's a critical step in designing, operating, and maintaining effective filtration systems.
Common Problems Related to Pore Size
While membrane filters are incredibly effective separation tools, their intricate pore structure also makes them susceptible to several operational problems. Many of these challenges, such as fouling, clogging, and the need for integrity testing, are intrinsically linked to the membrane's pore size and its interaction with the fluid being filtered.
Fouling: How Pore Size Affects Membrane Fouling
Fouling is arguably the most pervasive and significant challenge in membrane filtration. It refers to the accumulation of unwanted materials on or within the membrane pores, leading to a decrease in permeate flux (flow rate) and/or an increase in the transmembrane pressure (TMP) required to maintain flux. This accumulation essentially reduces the effective pore size and increases resistance to flow.
How pore size influences fouling:
- Smaller Pore Sizes, Higher Fouling Tendency: Membranes with smaller pores (UF, NF, RO) are generally more susceptible to fouling because they reject a wider range of substances, including smaller colloids, macromolecules, and dissolved organic matter that can deposit on the membrane surface or adsorb into the pores. The tighter structure offers more sites for interaction and less space for foulants to pass through.
- Pore Plugging: Particles or molecules larger than the membrane's pores will accumulate on the surface, forming a "cake layer." This layer acts as a secondary filter, adding resistance and reducing flux.
- Pore Blocking/Adsorption: Smaller foulants, particularly dissolved organic molecules, can adsorb to the internal surfaces of the pores or block the pore entrance, effectively reducing the pore diameter. This is often more difficult to clean than surface fouling.
- Biofouling: Microorganisms (bacteria, fungi, algae) can attach to the membrane surface and proliferate, forming a sticky biofilm. This biofilm can quickly cover pores, significantly impede flux, and even lead to irreversible damage if not managed effectively. Pore size doesn't prevent biological attachment but a denser membrane can limit penetration.
Fouling reduces filtration efficiency, increases energy consumption (due to higher pressure requirements), shortens membrane lifespan, and necessitates frequent cleaning or replacement, all of which add to operational costs.
Clogging: Issues and Prevention Strategies
Clogging is a severe form of fouling where the membrane pores become completely blocked, often by larger particles or aggregates, leading to a drastic or complete loss of flux. While fouling can be a gradual decline, clogging can be more sudden.
Issues related to clogging:
- Irreversible Damage: Severe clogging can make membranes impossible to clean, leading to premature replacement.
- Uneven Flow Distribution: Partially clogged membranes can lead to uneven flow across the membrane surface, potentially creating localized areas of higher pressure and stress.
- System Shutdowns: Frequent clogging necessitates system downtime for cleaning or membrane replacement, impacting productivity.
Prevention Strategies for Clogging:
- Effective Pre-treatment: This is the single most important strategy. Using coarser filters (e.g., cartridge filters, granular media filters) or even MF membranes as a pre-filter before UF, NF, or RO systems can remove larger suspended solids and reduce the load on the finer membranes.
- Appropriate Pore Size Selection: Choosing a pore size that is suitable for the feed water quality and the level of pre-treatment applied. Over-filtering (using too small a pore size for a given feed) will exacerbate clogging.
- Optimized Flow Dynamics: Operating at appropriate cross-flow velocities in tangential flow filtration (TFF) helps to sweep foulants away from the membrane surface, minimizing cake layer formation.
- Regular Cleaning Regimes: Implementing a schedule for chemical cleaning (Clean-In-Place or CIP) and/or physical cleaning (e.g., backflushing for MF/UF) to remove accumulated foulants before they become irreversibly clogged.
Integrity Testing: Ensuring Consistent Pore Size and Performance
Given the critical role of pore size in membrane performance, particularly in applications requiring absolute particle or microbial retention (e.g., sterile filtration), integrity testing is paramount. Integrity testing verifies that the membrane's pore structure remains intact and free from defects, cracks, or bypass channels that would effectively create larger-than-intended pores.
- Why it's Crucial: Even a single manufacturing defect or operational damage (e.g., from excessive pressure, chemical attack, or handling) can lead to a "pinhole" or tear. Such a defect bypasses the designed pore size exclusion, allowing contaminants to pass through, compromising the entire filtration process.
- Common Methods:
- Bubble Point Test: As discussed, this is a primary method. A drop in the bubble point pressure indicates a large defect.
- Diffusion Test: Measures the gas flow through the wetted pores at a pressure below the bubble point. An excessive flow indicates a defect.
- Pressure Hold Test: Measures the pressure decay over time in a sealed, gas-pressurized wetted filter. A rapid pressure drop suggests a leak.
- Forward Flow Test: Similar to the diffusion test, but measures total gas flow, which includes both diffusion and bulk flow through any large defects.
Integrity testing is routinely performed before and after critical filtration processes (especially in pharmaceuticals and sterile applications) and after cleaning cycles. It provides assurance that the membrane's effective pore size performance is maintained throughout its operational life.
In summary, managing problems related to membrane pore size, such as fouling and clogging, requires proactive strategies involving careful pre-treatment, optimized operation, and robust cleaning. Furthermore, regular integrity testing provides confidence that the membrane's crucial size-exclusion capabilities remain uncompromised.
Choosing the Right Membrane Filter
The journey from understanding what pore size means to grasping its diverse applications culminates in the critical task of choosing the right membrane filter for a specific need. This decision is rarely straightforward and involves a systematic assessment of several key factors to ensure optimal performance, efficiency, and economic viability.
Assessing Your Specific Filtration Needs
The first and most important step is to clearly define the objectives of your filtration process. Ask yourself:
- What is the desired outcome? Are you trying to:
- Clarify a liquid (remove turbidity)?
- Sterilize a solution (remove bacteria/viruses)?
- Concentrate a valuable product (e.g., proteins)?
- Remove dissolved salts or specific ions?
- Purify water to an ultrapure level?
- What is the required purity level? What is the maximum allowable concentration or size of residual contaminants? This will directly guide the required pore size. For example, a 0.45 µm filter might be sufficient for general clarification, but a 0.22 µm or tighter filter is needed for sterile filtration.
- What is the nature of the feed stream? Is it a liquid or a gas? What is its typical particulate load or dissolved solids content? Is it highly viscous or relatively thin?
- What is the required throughput (flow rate)? How much liquid or gas needs to be processed per unit of time? This influences not only the membrane type but also the total membrane surface area needed.
- What are the regulatory requirements? For applications in pharmaceuticals, food and beverage, or drinking water, there may be specific regulatory standards (e.g., FDA, USP, WHO) that dictate filter performance.
A clear understanding of these needs will narrow down the potential membrane types (MF, UF, NF, RO) and their corresponding pore size ranges.
Considering the Properties of the Fluid Being Filtered
Beyond the contaminants, the characteristics of the fluid itself play a significant role in membrane selection, particularly concerning membrane material compatibility.
- Chemical Composition:
- pH: The fluid's pH must be compatible with the membrane material. Some materials degrade rapidly in highly acidic or alkaline conditions.
- Presence of Solvents: Organic solvents can swell, dissolve, or severely damage certain polymeric membranes. Ceramic membranes or specific solvent-resistant polymers (e.g., PVDF) might be necessary.
- Oxidizers: Strong oxidizers (like chlorine) can damage many membrane materials, especially polyamide RO/NF membranes. Chlorine-resistant membranes or pre-treatment for chlorine removal may be required.
- Temperature: The operating temperature range must be within the membrane material's tolerance limits. High temperatures can cause membrane degradation or changes in pore structure. Conversely, very low temperatures can increase fluid viscosity, reducing flux.
- Viscosity: Highly viscous fluids require higher operating pressures or larger membrane surface areas to achieve desired flow rates, regardless of pore size.
- Fouling Potential: Assess the potential for the fluid to foul the membrane. Fluids high in suspended solids, colloids, dissolved organic matter, or microorganisms will require more robust pre-treatment, specific membrane materials, or effective cleaning strategies. Membranes with surface properties that resist adhesion (e.g., hydrophilic surfaces for aqueous solutions) can be beneficial.
Evaluating the Cost-Effectiveness of Different Membrane Types
The capital and operating costs associated with membrane filtration systems vary significantly depending on the chosen technology and its scale.
- Capital Expenditure (CAPEX):
- Membrane Cost: Finer pore membranes (RO > NF > UF > MF) are generally more expensive per unit area due to their complex manufacturing.
- System Components: Higher pressure operations (RO, NF) require more robust pumps, pressure vessels, and piping, increasing initial setup costs.
- Operational Expenditure (OPEX):
- Energy Consumption: Pumping costs are directly proportional to the operating pressure and flow rate. RO systems, requiring the highest pressures, have the highest energy consumption.
- Membrane Replacement: Lifespan varies by application, feed quality, and cleaning regimen. Replacing fine-pore membranes can be a significant recurring cost.
- Cleaning Chemicals and Procedures: The frequency and aggressiveness of cleaning required to combat fouling contribute to operating costs.
- Pre-treatment Costs: The level of pre-treatment needed to protect the membrane also adds to the overall operational budget.
It's crucial to perform a total cost of ownership (TCO) analysis that considers both initial investment and long-term operating expenses. Sometimes, investing in a slightly more expensive membrane with better fouling resistance or a longer lifespan can lead to significant savings in energy, cleaning, and replacement costs over the system's lifetime. Conversely, choosing an RO system when NF would suffice might be an unnecessary expenditure of capital and energy.
By carefully considering these interwoven factors—your filtration goals, the fluid's characteristics, and the economic implications—you can make an informed decision to select the membrane filter with the optimal pore size and properties for your specific application. This holistic approach ensures not just effective filtration, but also a sustainable and cost-efficient operation.
Still have question? simply contact hangzhou nihaowater, we would like to help.
 +86-15267462807
+86-15267462807




